WhiMSICAL study – on the way to 1000 participants and ‘big data’

The diagnosis, treatment, symptom and side-effect details of more than 400 people with Waldenström’s macroglobulanaemia (WM) from 18 countries are recorded on the WhiMSICAL study – the first global WM registry.
This ethically approved and secure database, that went live in June 2016, enables people with WM to directly contribute their own data to advance research in this field.
Dr Tohidi-Esfahani’s “big lofty goal” is for 1000 people with WM to be on the database, and as it grows “the data becomes more powerful and useful”.
Our aim is to get ‘big real-world data’ to help map quality of life (QOL) and treatment data, especially for the Bruton tyrosine kinase inhibitors such as ibrutinib (Imbruvica®), and to support the study’s ultimate goal; to improve QOL and survival.
“We’ve managed to break our 400-patient barrier – the cut-off point we needed to reach before collating the study findings in a manuscript,” said Dr Tohidi-Esfahani, who plans to submit the manuscript later this year for peer review and publication in a leading international haematology journal, and share the findings with the study’s participants.
Interim research findings have been presented at four international meetings, including this year’s European Hematology Association congress, in Amsterdam, and the ICML International Lymphoma meeting, in Switzerland, both in June.

Another important milestone is the completion of a validation study comparing data from WhiMSICAL with clinical data in an Australian cohort of 30 patients and Dr Tohidi-Esfahani said the result – a positive correlation of 83% or more for each data point – “was very pleasing”. This demonstrates the accuracy of the WhiMSICAL data and disputes the commonly held belief that patient-derived data is not very reliable for research purposes.
Andrew Warden, of Sydney, who has been living with WM since his diagnosis in 2003, and who played a key role in setting up the WhiMSICAL registry, was awarded the Judith May Volunteer Award by the International Waldenstrom’s Macroglobulinemia Foundation (IWMF) in Philadelphia in June.
“We are very proud of Andrew and his efforts to improve the breadth of information available on WM and his tireless energy in driving the research forward and improving the database,” said Dr Tohidi-Esfahani.

QOL questions included in survey
In October last year, a quality of life (QOL) questionnaire* was incorporated into the WhiMSICAL survey and over 200 patients have already answered these additional questions.
“Now we can compare the QOL scores of this WM cancer cohort to reference ranges for healthy controls available in the literature; that’s the power of this QOL questionnaire,” said Dr Tohidi-Esfahani.
“We’re starting to map QOL data to the treatments patients have had and this is especially important information that will guide funding bodies [such as the Pharmaceutical Benefits Advisory Committee] for the approval of drugs, and clinicians in their treatment decisions.”
An estimated 700-1000 Australians are living with WM. This figure is based on our incidence rate being similar to that in the U.S. (three per million population per year) and an average survival rate of up to 11 years from diagnosis.
No national registry for WM in Australia
Dr Tohidi-Esfahani said “no good data” exists on the exact number of WM patients in Australia as there is no nationally coordinated registry to record all diagnoses. This gap is due to Australia’s separate state health systems, where data [that is collected] is in silos and not entered into one database.
“To do that would be both difficult to run and costly,” said Dr Tohidi-Esfahani.
Attempts at gathering Australian WM data are being made through the Lymphoma and Related Diseases Registry, based at Monash University (Melbourne) and the Uncommon Lymphoma Registry that is part of the ALLG National Blood Cancer Registry and which is being established by the Australasian Leukaemia & Lymphoma Group.
“This registry – WhiMSICAL – is relatively very cost-effective and has ethical approval to capture data from patients anywhere in the world,” said Dr Tohidi-Esfahani.
When this story was published, 101 Australians had participated in the WhiMSICAL study – that’s about 10% of all Australians with this rare form of lymphoma.
“We would like more than 50% of all Australians with WM to join the database and it would be fantastic if we got closer to 100%,” said Dr Tohidi-Esfahani.
“It’s not difficult to participate, but it does take time and we are streamlining the process.
“Every individual only has to put in a small amount of time and if you are newly diagnosed, it takes even less time.
“This individual effort required is significantly less than the almost prohibitive amount of time that a clinical registry team would need to have such a large database; the power of the many achieves a lot of data and information for very little time and money.”
Number of unique front-line WM therapies worldwide “incredible”
Early analysis of the study data reveals that worldwide, 238 patients had 41 unique frontline combination therapies, which Dr Tohidi-Esfahani describes “as incredible”.
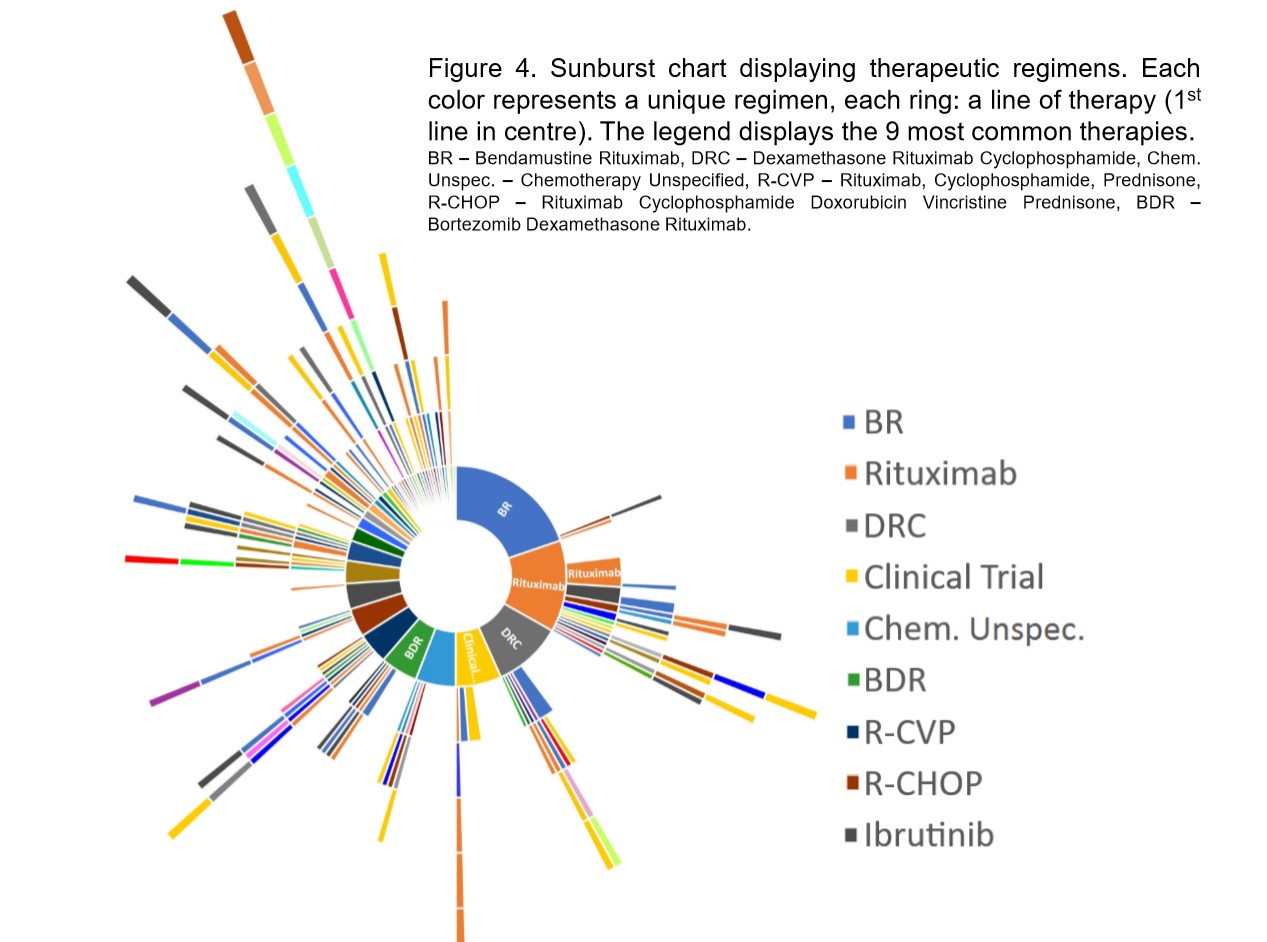
“To me, the fact there’s such a variety in the range of treatments people are getting outside of a clinical trial environment suggests a poor understanding of the best treatments available for WM.
“And the importance of this real-world data is to demonstrate the need for better consensus on what treatments to utilise and better education for patients and clinicians.
“In Australia, the data showed 17 first-line therapies in a cohort of 90 Australian patients (as at April 2019). That is less concerning, but still enlightening to the fact there isn’t well understood consensus on the guidelines here,” he said.
According to Australian clinical practice guidelines for the treatment of WM, introduced in January 2017 and international guidelines released in 2016, there are six different treatment combinations recommended for first-time treatment.
“It will be interesting, moving forward, to see the diversity in those more recently treated and whether there has been a significant improvement or not following publication of guidelines,” said Dr Tohidi-Esfahani.
“That will be a very important question to ask of the data we are generating.”
WhiMSICAL partners with patients
With a lot of research, Dr Tohidi-Esfahani said “patients are just voiceless participants”.
“But by forming a meaningful partnership with patients and having patient investigators on the study, patients get a say as to what questions are being asked in this research.
“They can make a significant contribution to the questions we should ask that are most important to them, the affected patient community, and some questions may be added or taken out.
“Since the beginning of the study, we’ve been adamant that every aspect of this research incorporates both the clinician and the patient investigator,” said Dr Tohidi-Esfahani.
“This is to ensure the ultimate aims and goals of the research are meeting the needs and providing answers to the patient community.”
* EORTC QLQ C30 has been used extensively and validated in cancer cohorts for 20 years. It is the longest and most comprehensive survey for comparing QOL over time, for different cancer cohorts and different treatments, with healthy controls. There are now ‘normal ranges’ of QOL, to compare to a cancer cohort.
Last updated on May 5th, 2021
Developed by the Leukaemia Foundation in consultation with people living with a blood cancer, Leukaemia Foundation support staff, haematology nursing staff and/or Australian clinical haematologists. This content is provided for information purposes only and we urge you to always seek advice from a registered health care professional for diagnosis, treatment and answers to your medical questions, including the suitability of a particular therapy, service, product or treatment in your circumstances. The Leukaemia Foundation shall not bear any liability for any person relying on the materials contained on this website.