Venetoclax moves us closer towards zero lives lost to blood cancer
We’re celebrating a breakthrough development for Australians with blood cancer after venetoclax was added to the Pharmaceutical Benefits Scheme (PBS) for people living with acute myeloid leukaemia (AML).
Affordable access to venetoclax means a new treatment possibility is now within reach for around 340 AML patients each year who might otherwise have had no other treatment options.
Intensive chemotherapy and a stem cell transplant are the current standard of care for AML but they’re often not suitable for many people because of their age or other health issues.
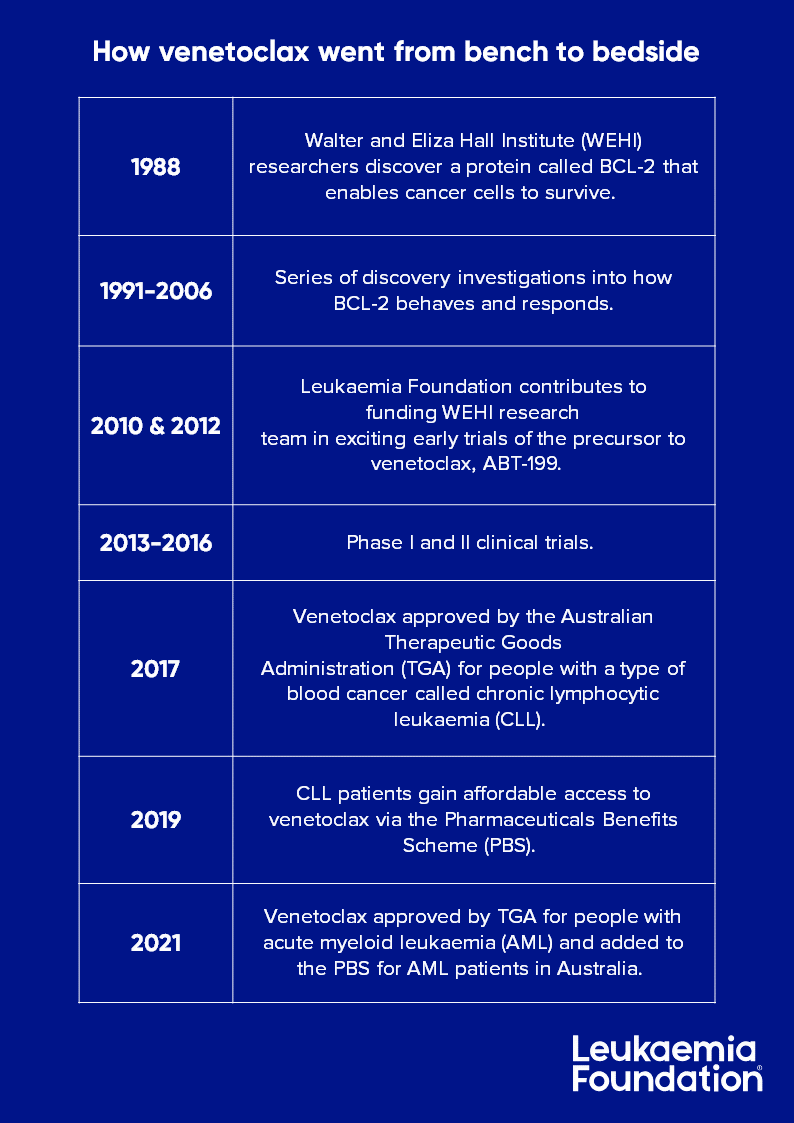
Thanks to our supporters, the Leukaemia Foundation was able to contributed to the early development of venetoclax in 2010 and 2012.
Today we stand proudly alongside the Australian blood cancer research community, who have been at the forefront of developing, trialling, and making this treatment available for chronic lymphocytic leukaemia (CLL) and now AML patients.
Giving more people access to this life-saving drug is the result of years of dedicated hard work by many teams of researchers, the contribution of big-hearted donors and the backing of Federal Government.
The Leukaemia Foundation will continue to advocate for the fast-tracking of blood cancer treatments like venetoclax, and support researchers working to further unlock the potential of the drug.
Blood cancer research is a long game but the outcomes, like venetoclax, can be life-changing and life-saving.
So we’ll continue to invest in new research projects and technologies that help more people with blood cancer and drive us closer to our goal of zero lives lost to blood cancer by 2035.
About Venetoclax
Venetoclax is a shining example of a discovery giving more people hope that surviving blood cancer is possible.
Venetoclax (Venclexta®) has been in development for over 30 years, tracing back to 1988 when Australian researchers first discovered a protein called BCL-2 that helps leukaemia cells survive.
Venetoclax targets and blocks the action of BCL-2. Blocking this protein helps to kill and reduce the number of cancer cells and may slow the spread of the disease.
The development of anti-cancer drug venetoclax was preceded by three decades of intense research, accelerating this life-saving treatment from bench to bedside.
About acute myeloid leukaemia (AML)
AML is a type of blood cancer that appears suddenly and grows quickly. AML is a devastating blood cancer that can rapidly overwhelm bone marrow without effective treatment.
AML occurs when immature white blood cells called blasts become cancerous. These abnormal blast cells are known as leukaemia cells.
In 2021, almost 5,000 Australians were diagnosed with leukaemia. In Australia, it is estimated that around 1,100 people are diagnosed with AML each year. AML becomes more common with age and mostly occurs in people aged over 65.
Need support? Whatever support, advice or information you might need, visit leukaemia.org.au/support.
For Sharn Coombes (pictured, left), the news about venetoclax being added to the PBS for people living with AML struck a very personal note.
Having lost her great-grandmother to AML, Sharn already had an understanding of the disease when her mother (pictured, centre) was diagnosed with polycythemia vera, or PV – a rare blood cancer that can progress to AML.
“Like many Australians, my mother would never be able to afford thousands and thousands of dollars for a treatment. It’s a very large amount of money, and to think that someone’s life is in the balance because of money is never acceptable.
“I’m very pleased this treatment is now going onto the PBS – it means that, should my mother’s condition progress, this drug will not only be available, but also affordable. That means a lot to me and my family.”
Last updated on February 22nd, 2022
Developed by the Leukaemia Foundation in consultation with people living with a blood cancer, Leukaemia Foundation support staff, haematology nursing staff and/or Australian clinical haematologists. This content is provided for information purposes only and we urge you to always seek advice from a registered health care professional for diagnosis, treatment and answers to your medical questions, including the suitability of a particular therapy, service, product or treatment in your circumstances. The Leukaemia Foundation shall not bear any liability for any person relying on the materials contained on this website.