John is positive his lymphoma is cured
It’s been 10 years since John Taylor’s last follow-up consultation with his haematologist and for the last eight years the long-term survivor of follicular lymphoma has thought of himself as cured.
The 91-year-old believes “having a very positive outlook” contributed to that outcome.
“I consider myself cured,” said John, who lives in a retirement village at Mandurah, south of Perth with his wife of 66 years, Eileen.
“But who knows what’s around the corner.”
Back in 2009, John said he wasn’t feeling unwell, but he went to the doctor about his spleen.
“It was getting larger and larger,” he said.
Initially, he was misdiagnosed with myeloma and told, “we can’t do much about it”. But a couple of months later the pain in John’s side was getting worse.
“I realised my spleen was getting larger and I needed to do something about it”.

Eileen took John to the hospital at Mandurah where the doctor on duty said, after seeing the results of an MRI, ‘I think you’ve got cancer John and you’re off to Fremantle’.
“That’s when we started on the road to recovery,” said Eileen.
John described the doctor he saw at the Fremantle hospital as “a wonderful, positive person”.
“He and I clicked straight away, and he put me on the right track. He did a fine needle aspiration and said I should go on treatment straight away that day.”
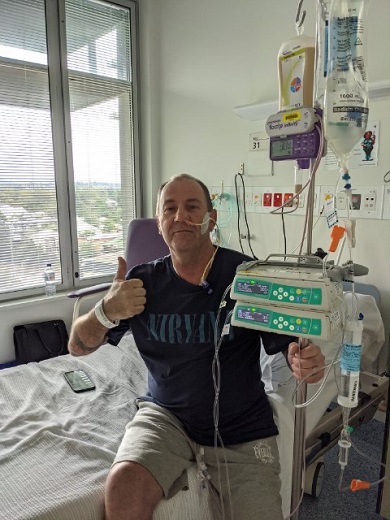
At the start of his lymphoma treatment, John spent the first fortnight in hospital and during that time he memorised the names of each of the 19 staff he encountered there.
“I had to fill my mind with something other than the treatment,” explained the retired engineer, who was 81 at the time.
“I was bored and up for a challenge. I involved myself by asking people their names, then applying my memory to it.
“And then, when I left hospital, I sent them a thank you card. I appreciated all their efforts and I named everyone, all 19 of those people who looked after me in some way.”
John spent the next three months going to and from Freemantle, where he had chemotherapy combined with the monoclonal antibody, rituximab (MabThera®) which he said, “seemed to do the trick”.
“They pumped all this juice in, and I got tea and a sandwich, and it was quite nice. I quite enjoyed it,” he said.
“My wife was the one who was driving [up and down from Mandurah] and trying to find a parking space!”
“The only time I got sick was the very initial treatment in hospital. The nurses were all garbed up in gear and they did take care with it. Apparently, it was very poisonous.
“But after that I was okay, I didn’t get sick at all.”
This is where Eileen added to the conversation.
“John’s making very light of all this, but he was very sick, went through the treatment like a breeze, and also got over it very well,” she explained.
“When he initially saw the specialist, the specialist said to me, ‘I knew John was going to be alright from the moment he walked in, because he had such a good attitude, a different attitude – some people have it and some don’t’.”
He certainly confirmed that a positive attitude was one factor in recovering, said John.
“I’d read that it’s [follicular lymphoma] an incurable disease, but I felt, well, this is my chance to hang in there, and that’s what we did.”
After completing his treatment in June 2009, John saw his treating haematologist initially once a month.
“He would say to me, ‘here’s my star performer’, because I was responding so well,” said John.

The check-ups went to every three months, before stretching out to six-monthly.
“Then he said he only needed to check on me once a year, and ‘if you have any trouble come back and see us’, and I’ve never had any trouble.
“About two years after that we went away on holiday, and it went well. I didn’t even think about it then, I just returned to my normal life and attitude.
“Since then, I haven’t had any reaction and I’ve had no need to go back and see anybody,” said John about his experience with lymphoma.
“But the only thing is, I’ve been plagued with skin cancer for years, and the skin specialists have a lovely time ripping skin off and putting bits back,” said John who has been dealing with skin cancer for about 40 years.
“He’s been very seriously ill in hospital with it,” said Eileen.
“He’s got great patches where they’ve taken skin away and he’s not a pretty sight really… he’s not the man I married!”
Talking about how they, as a couple, reacted to John’s subsequent lymphoma diagnosis, Eileen said, “we just hung in together”.
“I didn’t drop my bundle and say, ‘oh, this poor man, he’s never going to live’. I didn’t feel like that and John was very positive.”
John describes the Leukaemia Foundation’s support as “marvellous – the things that they’ll do, the books and information they give you, there’s no end of it”, and he was referred to the support group at Mandurah.
“There were a lot of people there who were a lot worse than me, and I felt, well, boy I’m lucky.
“It was good to talk to them because I felt it gave me a benefit and I hope it gave them some support too.”
John says the best thing he ever did was get married.
“That really changed my life and direction. Togetherness and support – that makes a big difference.”
And the Taylors are blessed to have a son and daughter, and three grandchildren.
John and Eileen walk regularly, they have a good diet, don’t smoke, and do enjoy a sherry most nights, except the nights they play bridge. They also play croquet and are part of a computer group.
“We help people get over their computer troubles, and I quite enjoy that,” said John, who still drives.
He also helps his son out with bits and pieces and the latest project was organising a solar system to be installed on the roof of his son’s house.
“I like that sort of work, to have a project and get on with it. And I hope to do that for another year or two.”
For others, John has some simple advice: “be positive, don’t give in”.
“And what I say to the Leukaemia Foundation and the 19 people who looked after me in hospital – you gave me a new life.”



 Professor Andrew Wei, haematologist at the Alfred Hospital (Melbourne), said magrolimab “was a potential important advance for patients with MDS” and “a treatment option with a highly novel mechanism of action”.
Professor Andrew Wei, haematologist at the Alfred Hospital (Melbourne), said magrolimab “was a potential important advance for patients with MDS” and “a treatment option with a highly novel mechanism of action”.




 Leukaemia Foundation support
Leukaemia Foundation support