Myelodysplastic neoplasms (MDS)
What is MDS?
Myelodysplastic neoplasms (MDS) are a group of blood cancers which all affect the production of normal blood cells in the bone marrow. MDS occurs as a result of a mutation (or change) in one or more of the genes that control blood cell development. This change or changes results in the abnormal growth of blood stem cells.

In MDS, abnormal bone marrow stem cells (called blast cells) produce increased numbers of immature blood cells. These cells do not grow properly and often die prematurely. This results in lower numbers of:
- mature red blood cells
- white blood cells
- platelets
The blood cells that do survive are often of poor quality, are abnormal in shape (dysplastic) and are unable to function properly. This means that people with MDS often have a very active bone marrow but a low number of circulating blood cells. Without enough red blood cells, white blood cells and platelets you can become fatigued, more susceptible to infections, and to bleeding and bruising.
While MDS can occur at any age, most cases develop over the age of 60. MDS can occur very occasionally in children. It’s difficult to be sure of the exact number of people who have MDS. This is because in many cases it develops slowly and people don’t have any symptoms for a long time.
Causes of MDS
In most cases, there is no specific cause of MDS. MDS is either:
- primary, or de novo – where there is no known cause
- secondary, or treatment-related – where a person diagnosed with MDS has had prior chemotherapy and/or radiation therapy. Only 5-10% of people with MDS have treatment-related disease.
Why defects occur in the bone marrow and cause MDS in a particular person at a particular time is usually unknown.
There are some factors that may increase the risk of developing MDS:
- Ageing – the risk of developing genetic mutations increases with age
- Exposure to high levels of some environmental chemicals, especially benzene and petroleum products
- Exposure to chemicals in tobacco smoke
- People previously treated for cancer or other conditions with chemotherapy are at an increased risk of developing what is called secondary or treatment related MDS
- Previous radiation therapy, or accidental exposure to high levels of environmental irradiation
- People with certain congenital disorders such as Bloom’s Syndrome, Down’s Syndrome, Fanconi anaemia and neurofibromatosis can have unstable genes. They are more at risk of developing mutations that cause MDS
Symptoms of MDS
Many people in the early stages of MDS have no symptoms at all and it is picked up accidentally during a routine blood test. In other cases, people go to their doctor because they are experiencing some troubling symptoms. The types of symptoms that people experience depend on how severe their disease is and the type of blood cell that is most affected.
The most common symptoms of MDS:
Anaemia, caused by a lack of red cells:
Abnormal white cell function, often with low white cell counts, causes:
Abnormal platelet function, often with low platelet counts, causes:
Some terms you may encounter:
Anaemia
Leukopenia
Thrombocytopenia
Pancytopenia
Many people with MDS have a combination of symptoms. This is because the production of all of the blood cell types may be affected by the disease. Some of these symptoms may also be seen in other illnesses, including viral infections. It is important to see your doctor if you have any symptoms that do not go away. You should be examined and treated if necessary.
Diagnosis of MDS
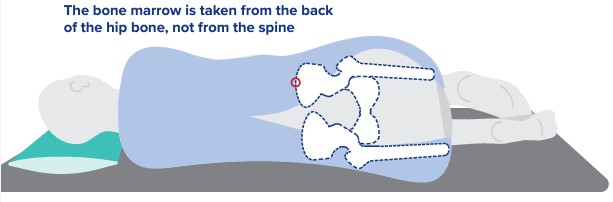
- medical history and physical exam
- blood tests – full blood count (FBC), kidney and liver function, electrolytes
- bone marrow biopsy
- genetic tests (cytogenetics)
Types of MDS
There are different types of MDS which vary in how normal blood cell production is affected. People with mild disease are often anaemic, or they might have a low white blood cell or platelet count. In many cases people may have few, if any, troubling symptoms. In more severe cases, when these blood cells are low people have more symptoms.
The current World Health Organisation’s classification system recognises several major subtypes of MDS. Knowing the exact type of MDS you have is important because it helps your treatment team decide the best course of treatment.
The World Health Organization (WHO) classification system (2022)
The WHO classification system uses the new term, Myelodysplastic Neoplasms (MDS). This system divides MDS into 2 groups:
MDS with defining genetic abnormalities
MDS morphologically defined
Your treatment team will discuss your type of MDS and your treatment options.
Prognosis of MDS
The risk of your MDS progressing into acute myeloid leukaemia (AML) and your life expectancy can be calculated by your treatment team. The score is calculated using the International Prognostic Scoring System – Molecular (IPSS-M) risk calculator. Your treatment team will input your bone marrow blast (immature) cell count, number and types of blood cell types affected, and number and type of genetic abnormalities and mutations.
International Prognostic Scoring System – Molecular (IPSS-M) risk calculator
Treatments for MDS
Your haematologist will recommend treatment based on:
- the type of MDS you have
- your age
- your general health
- your prognosis
- your wishes
Watch and wait
Supportive care
Targeted therapy
Chemotherapy
Stem cell transplant
Myelodysplastic/myeloproliferative neoplasms (MDS/MPN)
These are a group of rare cancers that have characteristics of both
- myelodysplastic (abnormal bone marrow cells producing too few blood cells) and
- myeloproliferative (abnormal bone marrow cells producing too many blood cells) neoplasms.
Myelodysplastic/myeloproliferative neoplasms may progress to acute leukaemia. There are generally 3 types:
- Juvenile myelomonocytic leaukaemia (JMML) is an uncommon childhood blood cancer that has overlapping features of myelodysplastic/ myeloproliferative neoplasms (MPN)
- Chronic myelomonocytic leukaemia (CMML) is similar to JMML but commonly occurs in older adults
- Atypical chronic myeloid leukaemia (aCML) has been renamed in the fifth addition of the WHO classification to MDS/MPN with low neutrophils
Treatment depends on the characteristics of each blood cancer.
References
Molecular International Prognostic Scoring System for Myelodysplastic Syndromes | NEJM Evidence IPSS-M Risk Calculator (mds-risk-model.com) The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms (mll.com) The New WHO Classification 2022 | MLL Cells | Free Full-Text | Molecular Drivers of Myelodysplastic Neoplasms (MDS)—Classification and Prognostic Relevance (mdpi.com) Allogeneic Hematopoietic Cell Transplantation Improves Outcome in Myelodysplastic Syndrome Across High-Risk Genetic Subgroups: Genetic Analysis of the Blood and Marrow Transplant Clinical Trials Network 1102 Study – PMC (nih.gov) Myelodysplastic disorders | eviQMore information
Allogeneic stem cell transplants – Leukaemia Foundation factsheet Allogeneic stem cell transplants Myelodysplastic neoplasms (MDS) information booklet Optimal Care Pathways for patients Acute myeloid leukemia (AML) Acute myeloid leukemia (AML)How we can help
Online Blood Cancer Support Service Support services Online support groups and webinarsMDS stories
MDS patient stories and research newsLast updated on September 3rd, 2024
Developed by the Leukaemia Foundation in consultation with people living with a blood cancer, Leukaemia Foundation support staff, haematology nursing staff and/or Australian clinical haematologists. This content is provided for information purposes only and we urge you to always seek advice from a registered health care professional for diagnosis, treatment and answers to your medical questions, including the suitability of a particular therapy, service, product or treatment in your circumstances. The Leukaemia Foundation shall not bear any liability for any person relying on the materials contained on this website.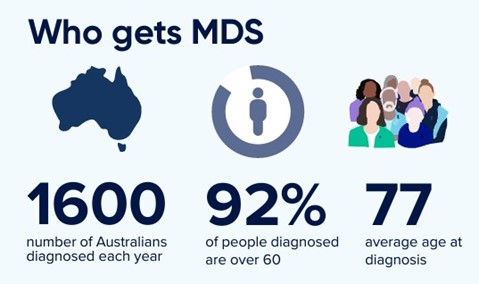

 Supportive care controls symptoms of MDS and side effects. Supportive care aims to improve quality of life. It is frequently used for older people or those with other health problems. This group of people are often unable to tolerate the stronger treatments for MDS. Supportive care does not aim to treat the disease. It can help with symptoms such as shortness of breath, bruising or bleeding.
Supportive care controls symptoms of MDS and side effects. Supportive care aims to improve quality of life. It is frequently used for older people or those with other health problems. This group of people are often unable to tolerate the stronger treatments for MDS. Supportive care does not aim to treat the disease. It can help with symptoms such as shortness of breath, bruising or bleeding. Targeted therapy directly targets the mutations/changes inside the blood
Targeted therapy directly targets the mutations/changes inside the blood  Low intensity chemo may be given to people with intermediate II or high risk MDS.
Low intensity chemo may be given to people with intermediate II or high risk MDS. People with MDS who have a high risk of progressing to
People with MDS who have a high risk of progressing to